Queensland pulls AstraZeneca vaccine following more cases of blood clots
05/27/2021 / By Ramon Tomey
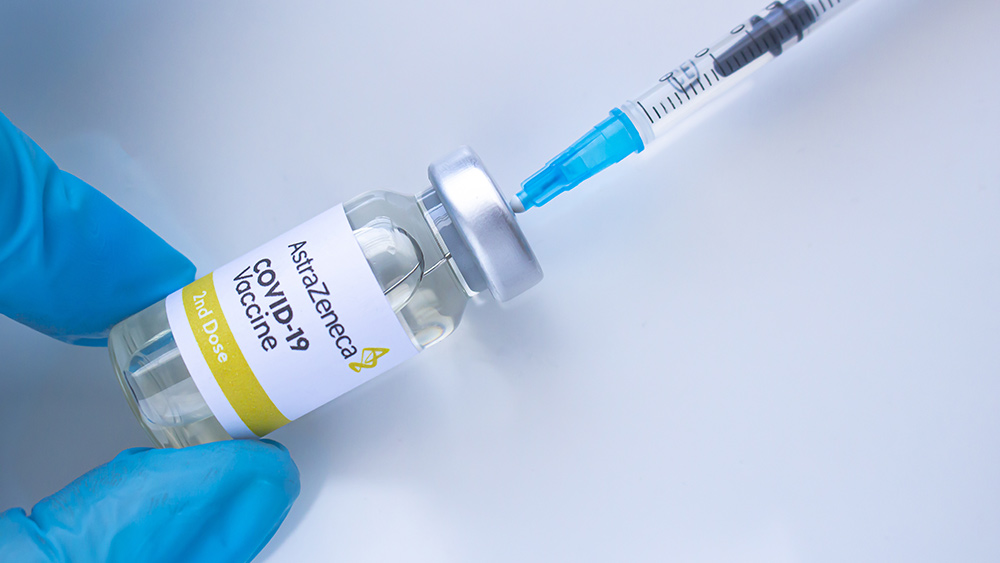
The state government of Queensland in Australia announced that it will not offer Wuhan coronavirus (COVID-19) injections using the AstraZeneca vaccine. Its decision came after health authorities in the country uncovered six more cases of blood clots linked to the vaccine candidate. Instead, the state will wait for the arrival of more vaccine doses from other manufacturers before resuming.
The vaccine made by the British drug manufacturer is still administered in the state by doctors to people above 50 years old. However, the state’s government said that it will not use the AstraZeneca vaccine in mass vaccination centers for inoculating Australians. Queensland Premier Annastacia Palaszczuk said such a mass vaccination hub similar to those in New South Wales and Victoria is being planned toward the end of 2021.
“Queensland is a big state, [and] it is so decentralized. [So] much planning is happening at the moment for that final quarter of the year. [When] we have more supply [of] Pfizer [and] Moderna, [vaccination] is going to ramp up – and I know that Queenslanders are going to go out there in droves when we have all that supply ready,” Palaszczuk said.
The Queensland premier’s remarks followed Australian authorities discovering six new cases of blood clots connected to the AstraZeneca vaccine. The Therapeutic Goods Administration (TGA), which regulates vaccines in the Land Down Under, said on May 20 that four cases were confirmed while the other two were deemed probable. With the six new cases, Australia now has a total of 24 cases of blood clots out of the 2.1 million AstraZeneca doses administered there.
TGA continued: “Overall, 21 of these [24 total] cases are considered confirmed and three are considered probable. Three of the four cases reported as probable in last week’s report have since been confirmed as [blood clots and low platelet count.] The other case remains under investigation.”
The new blood clot cases were recorded from all over Australia
A 57-year old woman and a 79-year old man were the cases recorded from Victoria state. Queensland state reported an 18-year-old woman reportedly suffered from the clots. Meanwhile, authorities still sought more information about two women aged 71 and 87 who reported the adverse effects.
A 53-year-old man from South Australia – the sixth recent case – is currently in intensive care in Adelaide. The man is believed to be the state’s first confirmed patient who suffered blood clots and low platelet count after vaccination. South Australia Chief Public Health Officer Nicola Spurrier said the man received his first dose on May 4. He was admitted two weeks later due to severe abdominal pain.
Spurrier added that the man’s case fell within the expected range of adverse reactions – about one in 100,000 – given that about 150,000 AstraZeneca vaccine doses had been administered in the state. “It is a rare occurrence, but because of the number of vaccines that we’re giving across our whole community – it was not unexpected for this to occur.”
Meanwhile, Federal Deputy Chief Medical Officer Michael Kidd acknowledged the blood clots and low platelet count linked to the AstraZeneca COVID-19 vaccine. But he stresses that the chances of it happening are “very small.” He said: “Cerebral venous thrombosis is a very rare disorder that has previously not been known to be associated with vaccination.” (Related: Australian man dies of a massive BLOOD CLOT following coronavirus vaccination.)
Nevertheless, he warned of possible headaches as a side effect of the COVID-19 vaccine. “People should be particularly alert to severe persistent headaches occurring four to 20 days after vaccination,” Kidd said. The federal health official added that these headaches “are different [from] the usual pattern of headaches” and advised against using over-the-counter painkillers for them.
Australian government officials still insist on the AstraZeneca coronavirus vaccine’s effectivity
Prime Minister Scott Morrison defended the AstraZeneca vaccine on May 20 and exhorted Australians over 50 years old to get inoculated with it. He said that most people he knew – including his wife, mother and mother-in-law – had been vaccinated with it alongside many others.
A day before Morrison made his comments, Health and Aged Care Minister Greg Hunt suggested that people could wait for the Pfizer and Moderna mRNA vaccines to arrive in case they did not want the AstraZeneca vaccine. Hunt himself received the AstraZeneca vaccine in early March alongside former Prime Minister Julia Gillard. (Related: Aussie Health Minister Greg Hunt hospitalized after getting the AstraZeneca coronavirus jab.)
Back in January, Australia’s Federal Chief Medical Officer Paul Kelly stood by the British company’s vaccine. “The AstraZeneca vaccine is effective, safe, and … high-quality. It will be available as soon as the TGA gives its tick,” he said. The Australian regulator granted provisional approval to the vaccine in February 2021.
Australian Labor Party Deputy Leader Richard Marles ultimately insisted the government should not be encouraging people to wait. “It’s really important that people over the age of 50 get vaccinated with [the] AstraZeneca [vaccine] right now,” he said.
Visit VaccineDamage.news to read more reports about the AstraZeneca COVID-19 vaccine and blood clots linked to it.
Sources include:
Tagged Under: adverse reaction, AstraZeneca, Australia, Big Pharma, Blood clots, coronavirus vaccine, covid-19 pandemic, low platelet count, Queensland, vaccine damage, Wuhan coronavirus
RECENT NEWS & ARTICLES
COPYRIGHT © 2017 IMMUNIZATION NEWS